Guiding Device For Vascular Cell Seeding
Context & Objectives
This project was conducted as part of the PJT course at Arts et Métiers ParisTech. In collaboration with biomedical researchers, we aimed to design a magnetic guidance device that enables targeted vascular cell deposition within the carotid artery. This approach addresses complications from diseases like sickle cell anemia, by enabling precise delivery of therapeutic cells to specific zones of the vascular wall.
The concept, initially proposed by Dr. Saskia Eckert, involves guiding a magnetically-labeled cell through the bloodstream and promoting its adhesion to pre-defined regions of the carotid. Our role was to deliver a fully operational, lab-scaled in vitro guidance system with a precise anatomical reconstruction and electromagnetic control.
- Pathology addressed: Sickle cell anemia (drépanocytose)
- Goal: Enable magnetic guidance and adhesion of a functionalized cell
- Use case: In vitro experimental setup (no clinical application)
- Main collaborators: Dr. Eckert (biomedical lead), Prof. Segonds (design methodology)
Design Requirements
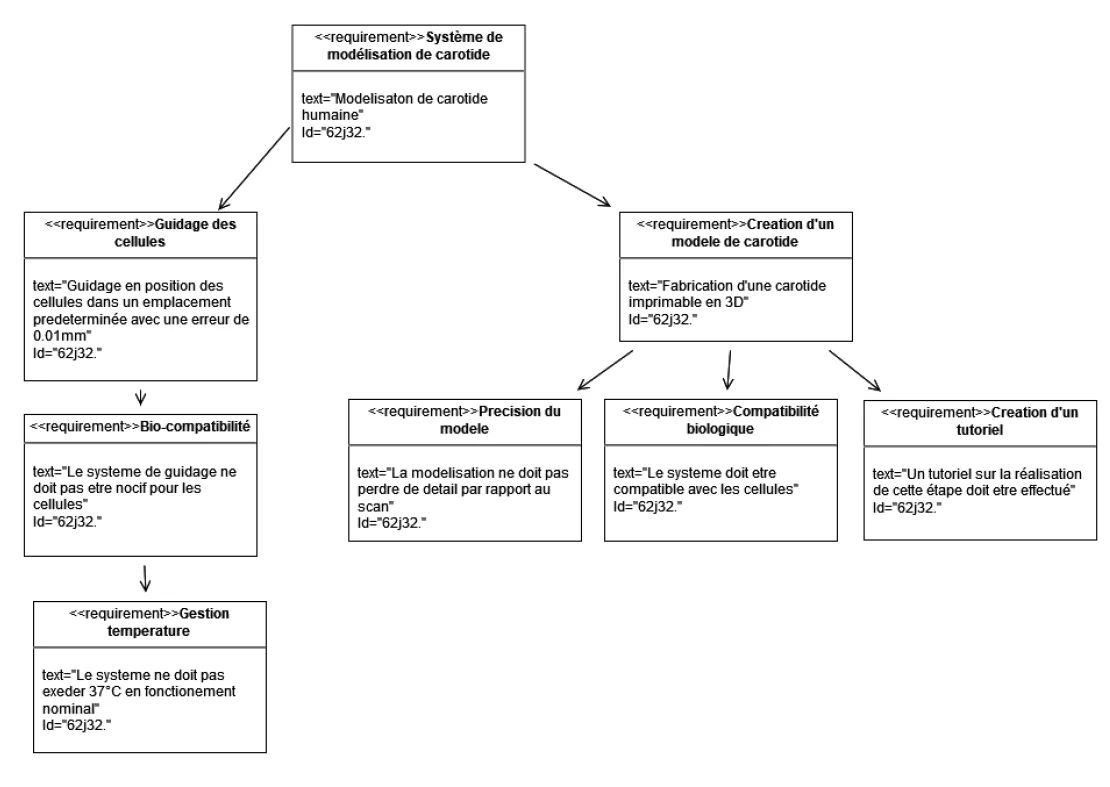
To meet the technical and biomedical constraints of the project, we established a detailed set of requirements across four categories:
- Biomedical constraints: No in vivo testing; cell viability must be preserved; temperature < 37°C; no mechanical stress from the magnetic field.
- Magnetic constraints: Adjustable field intensity and direction; magnetic force ≥ 0.1 pN; uniform cell adhesion; reproducibility of experiments.
- Manufacturing constraints: Anatomical fidelity of the carotid; 3D print precision ±50 µm; biocompatible materials; standard fluidic ports (Ø 3.2 mm); reproducible protocol.
- Experimental constraints: In vitro bench testing; live monitoring of temperature, magnetic field, flow rate, and pressure.
These constraints were formalized using a SysML requirement diagram, built with Visual Paradigm. It helped structure the interdisciplinary expectations between biomedical and mechanical domains.
Magnetic Targeting Strategies Benchmark
During the early design phase, we compared different approaches to guide magnetically-labeled cells within vascular structures. The selected strategy had to ensure both precision and experimental feasibility.
| Strategy | Precision | Biocompatibility | Implementation |
|---|---|---|---|
| SPIONs (internal injection) | Low | Good | Complex |
| Magnetic Antibody Conjugates | Medium | Good | Complex |
| External Static Magnets | High | Good | Moderate |
| Magneto-immunotherapy | High | Variable | Difficult |
We finally selected external static magnets, which offer strong magnetic gradients without interfering with cell biology or requiring internal modification.
Concept & Feasibility
Several strategies for magnetic cell targeting were considered during the early design phase. After benchmarking approaches such as SPIONs, antibody-nanoparticle conjugates, and magneto-immunotherapy, we selected external magnetic targeting for its precision and adjustability.
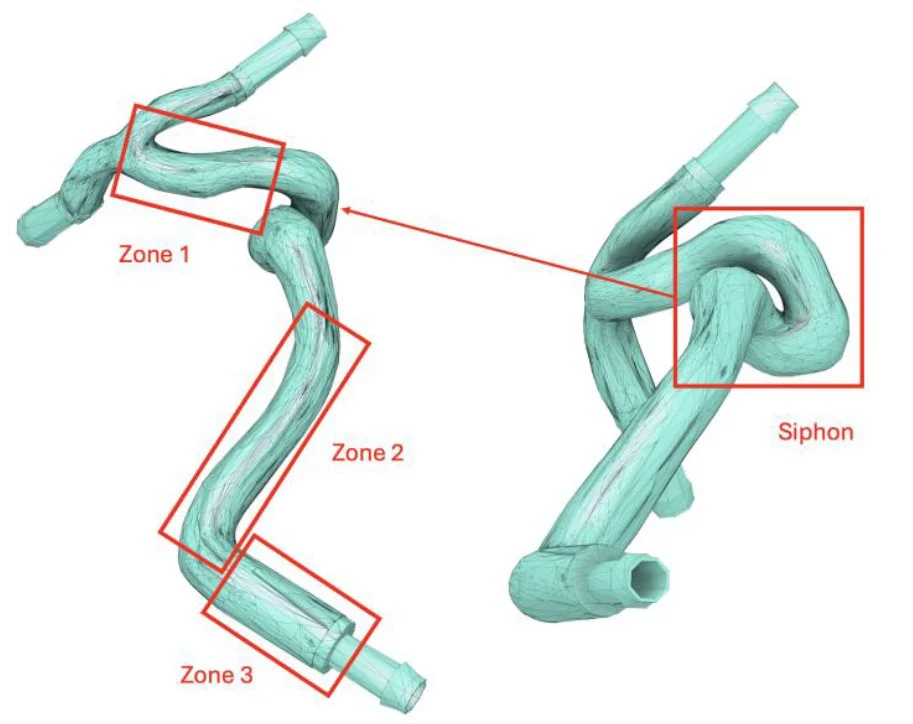
Using medical scan data, we divided the carotid geometry into three target zones and identified the bifurcation as the most viable region for magnetic deposition. Zones like the siphon were excluded due to their geometric complexity and difficult access.
- Feasible targets: Zones 1, 2, 3 and bifurcation
- Excluded regions: Siphon and distal arteries (ACA, MCA)
- Selected strategy: Static external field, optimized for close-proximity guidance
This feasibility analysis ensured realistic boundaries for the magnetic design while preserving the project's core objective.
Design & Development
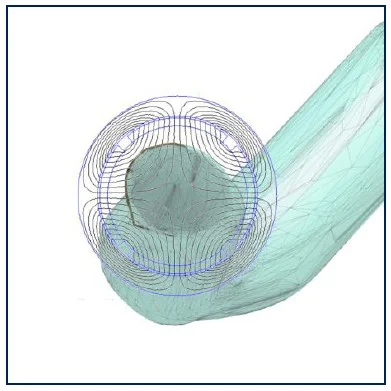
The system design combined analytical modeling of magnetic forces with anatomical fidelity. Based on Stokes' drag and a simplified force balance, we computed the minimum force required to overcome blood viscosity and achieve adhesion to the arterial wall.
- Target magnetic force: 2.5 pN (validated by literature)
- Distance to vessel wall: ~1.44 cm from magnet to cell
- Model assumptions: laminar flow, spherical particle, neglect of gravity
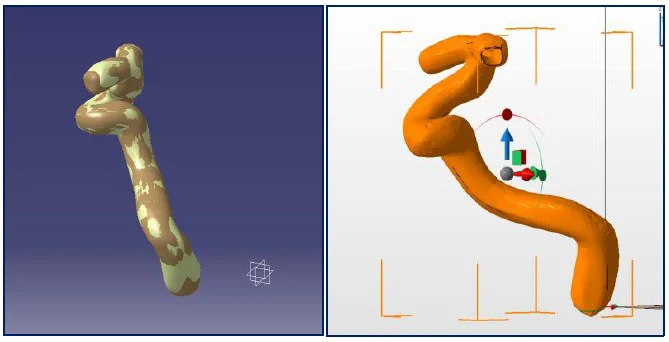
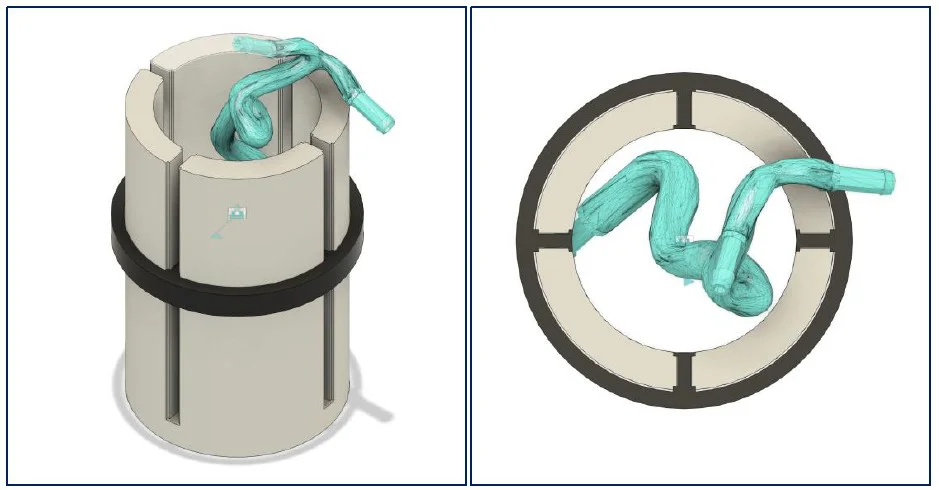
In parallel, the carotid geometry was reconstructed from DICOM using CATIA and NetFabb. A 1 mm shell offset was created to define printable geometry while preserving the detailed inner wall. Fluidic ports (Ø 3.2 mm) were added.
Validation & Optimization
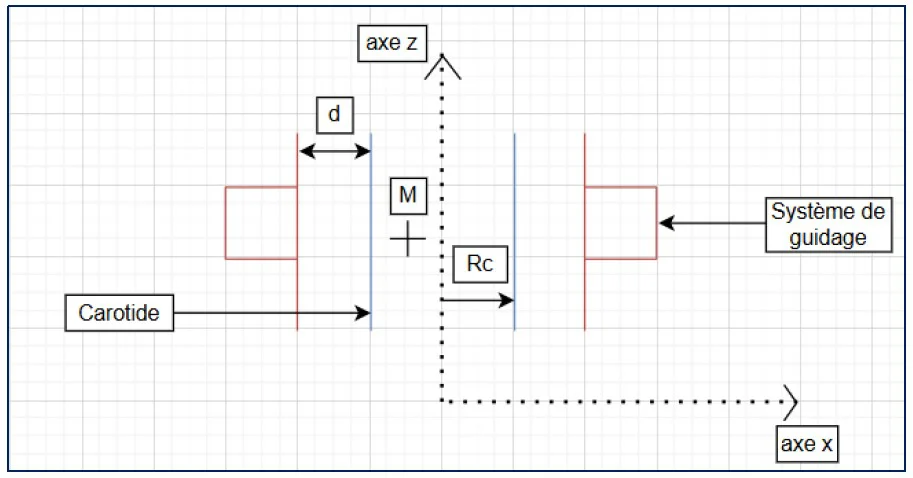
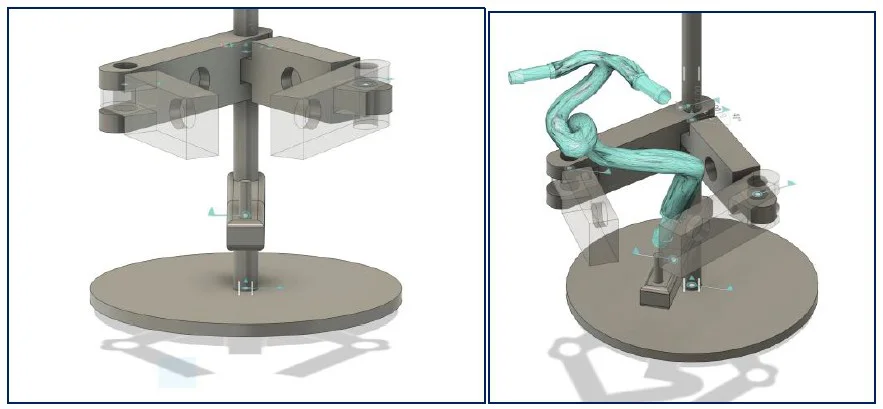
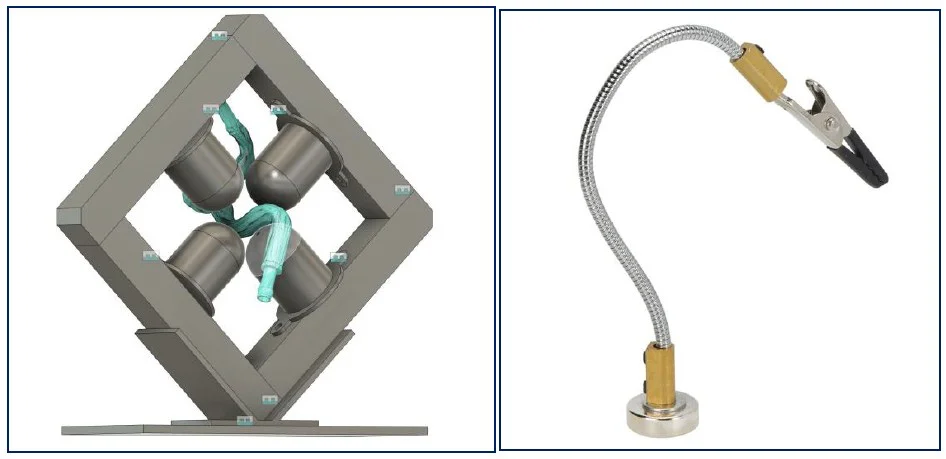
Three configurations were designed to hold the magnets and maintain the carotid during lab tests. Each solution aimed to achieve reliable positioning while preserving access to the vessel for perfusion and imaging.
- Solution 1: Four articulated arms – flexible but difficult to calibrate
- Solution 2: Sliding magnet holders along a cylinder – geometry too irregular
- Solution 3 (chosen): Fixed square frame with articulating arm – simple and stable
Solution 3 was adopted for its simplicity and reproducibility. Field distribution was validated across zones 1–3, confirming sufficient magnetic gradients for cell guidance.
Discussion
The AM-Biopart methodology helped organize interdisciplinary tasks and ensure medical constraints were incorporated early. While the prototype met functional expectations, several limitations were identified:
- Non-uniform cell deposition was observed with static magnetic fields
- Siphon targeting remains unresolved due to geometric constraints
- In vivo translation would require extensive validation and biological coordination
Future improvements could include mobile or rotating magnets for uniform adhesion, and automated field mapping across the full vessel.
Conclusion
This project led to the successful conception of a vascular cell guidance system based on magnetic field control and 3D-printed anatomical models. Key milestones included:
- Definition of requirements and choice of AM-Biopart methodology
- Magnetic modeling and feasibility analysis
- Digital reconstruction of the carotid and printable design
- Prototype design and magnetic validation for in vitro use
This prototype lays the groundwork for future iterations involving dynamic guidance, enhanced biological compatibility, and experimental seeding trials.
References
- INSERM. Drépanocytose, la maladie génétique la plus présente en France. Available at: inserm.fr (accessed Dec 2, 2024).
- Hôpital Robert-Debré AP-HP. Qu’est-ce que la drépanocytose ? Available at: aphp.fr (accessed Dec 4, 2024).
- Eckert, S., Vedel, P., Nguyen-Peyre, K.-A., Kassasseya, C., Belkeziz, N., Bartolucci, P., & Segonds, F. The creation of an in-vitro model – an interdisciplinary challenge, internal communication (accessed Jan 2, 2025).
- Perea, H., Aigner, J., Hopfner, U., & Wintermantel, E. Direct magnetic tubular cell seeding: a novel approach for vascular tissue engineering. Available at: mediatum.ub.tum.de (accessed Jan 5, 2025).
- Griffiths, D. J. Introduction to Electrodynamics, 4th edition. Addison-Wesley, 2012. Available at: hansandcassady.org (accessed Dec 28, 2024).
Acknowledgments
I would like to thank my teammates Vincent Debernardi, Nacim Fahim, Manila Lacroix, Félix Revillet, Léon Schulz and Xunzhaoyang Yin for their collaboration and dedication throughout this project,
as well as Dr. Saskia Eckert and Prof. Frédéric Segonds (LCPI - ENSAM) for their guidance.